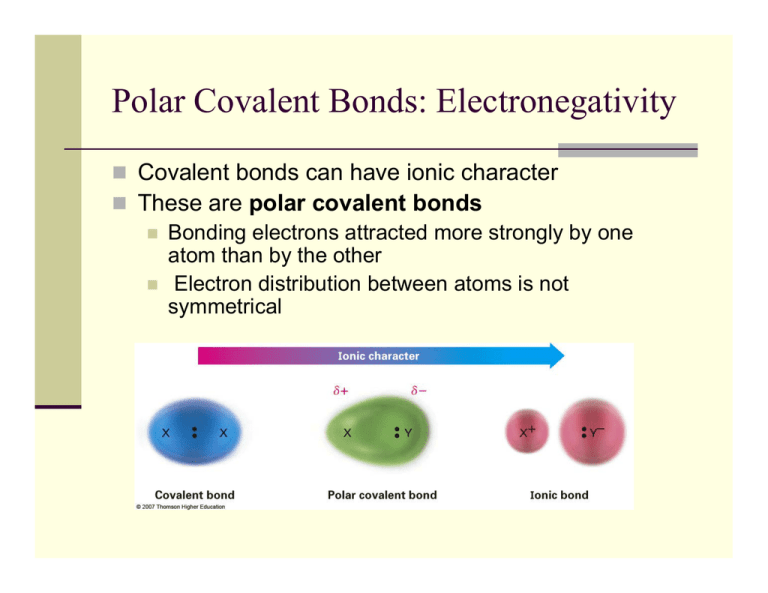
A chemical bond in which two atoms. A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed.

Polar Covalent Bond Definition And Examples
A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms.

. The charge of the electric dipoles is less than a full unit charge so they are considered partial. A bond formed that is half ionic and. A chemical bond in which two atoms share one or more pairs of electrons that hold them together2.
This causes the molecule to have a slight electrical dipole moment where one end is slightly positive and the other is slightly negative. A covalent bond formed with electrons that are shared equally B. A covalent bond formed with unequal sharing of.
What is the best definition of polar covalent bond. Polar Bond Definition. A polar bond is a covalent bond in which there is a separation of charge between one end and the other Examples include most covalent bonds.
A covalent bond formed with unequal sharing of electrons C. A bond formed that. Tagged with chemistry covalentbonding.
A covalent bond formed with electrons that are shared equally. A bond formed that is half ionic and half covalent. It is also defined as the bond which is formed due to the unequal sharing of electrons between the atoms.

0 Comments